A leader in gene therapy for musculoskeletal diseases
We develop life-changing medicines for highly prevalent conditions affecting millions of people.

Genascence was founded in 2017, with technology licensed from two leading U.S. research institutions: Mayo Clinic and University of Florida.


Our Approach
Interleukin 1 (IL-1), a potent inflammatory molecule, plays a central role in the pathophysiology and development of osteoarthritis (OA). Left unchecked, IL-1 causes inflammation and contributes to the major symptoms of OA – including pain – and causes cartilage destruction leading to joint degeneration in OA patients.
Genascence is developing a genetic medicine that is injected directly into the affected joint. It is based upon a gene therapy vector carrying a protein, IL-1 receptor antagonist (IL-1Ra) that transduces synovial cells, which line the joint capsule and chondrocytes that reside deep inside the cartilage. These cells become a biofactory that produces IL-1Ra, which blocks pathologic IL-1 signaling, returning it to physiological levels.
As depicted in the image below, when IL-1 binds to its IL-1R1 receptor, it recruits the IL-1 accessory protein (IL-1RAcP) and activates downstream signaling of numerous pathways leading to inflammation. When IL-1Ra binds to IL-1R1, the receptor chain is prevented from forming a receptor complex with IL-1RAcP. This inhibits the binding and signaling of IL-1, thus preventing the downstream inflammatory effects.
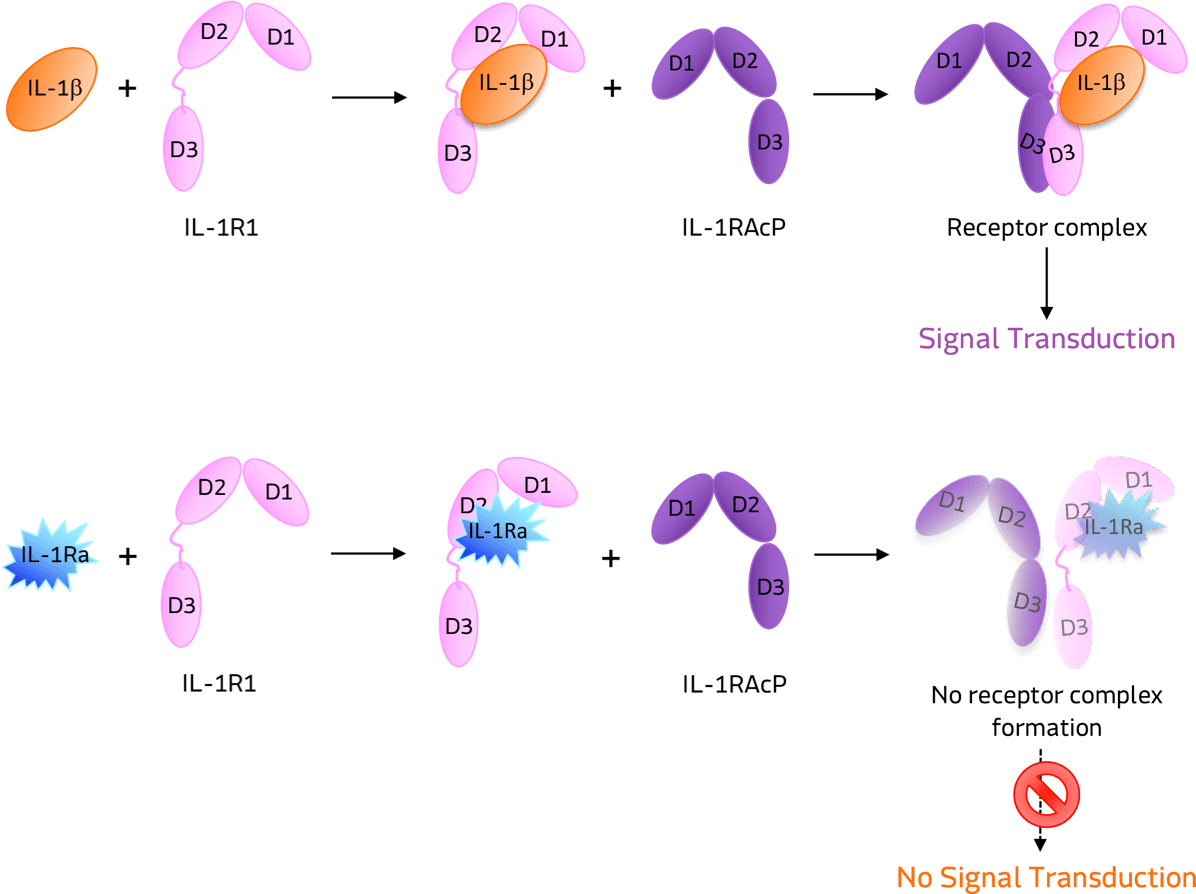
“We have long been interested in the molecular mechanisms that cause joint degeneration. Interleukin 1 is known to cause inflammation and cartilage destruction, and by blocking it, we hope to benefit patients with osteoarthritis. This could be revolutionary for patients suffering from OA.”
Prof. Christopher H. Evans
Professor Mayo Clinic and Genascence Co-founder

OUR LEAD PROGRAM: GNSC-001
GNSC-001 is a genetic medicine – a recombinant adeno-associated virus vector carrying a coding sequence for interleukin-1 receptor antagonist (IL-1Ra), a potent inhibitor of IL-1 signaling. GNSC-001 is administered as a one-time intra-articular injection, which is a straightforward procedure performed in a physician’s office. GNSC-001 is designed to offer long-term, sustained inhibition of IL-1 following a single injection.
GNSC-001 is currently being developed for OA of the knee. In pre-clinical studies, a single treatment with GNSC-001 lasted beyond one year and was able to improve both, symptoms and joint structure in a large animal model.
OSTEOARTHRITIS OF THE KNEE
Osteoarthritis (OA) of the knee occurs when the cartilage in your knee joint breaks down. These structural changes in bone within the joint makes your knees hurt, become stiff and swollen, contributing to pain, disability, and loss of joint function.
Age is a major risk factor for knee OA, but young people can get it as well. In some individuals, it may be hereditary; for others, knee OA can result from injury or infection or from being overweight.
Clinical Trials
Clinical trials play a critical role in the development of new therapies for patients in need. In the search to understand, prevent, and treat severe diseases, Genascence is committed to sharing information about clinical trials and making study results publicly accessible. We are committed to working toward a clinical trial infrastructure that addresses health disparities while closing the gap in clinical trial diversity to better reflect the intended treatment population.
For information about U.S. trials including information about disease area, study type, location, contact information, and recruitment status, visit ClinicalTrials.gov.
Note: Information provided on ClinicalTrials.gov should be used in conjunction with advice from healthcare professionals.
Expanded Access
Clinical Trials
Our goal, whenever possible, is to encourage patients to participate in our clinical trials. Clinical trials are well-controlled voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of investigational drugs. Participants in clinical trials are closely monitored and receive quality care and additional attention from clinical trial staff in all aspects of their disease, and therefore clinical trials often provide the safest opportunity for people to access an investigational drug before it is approved by a regulatory agency.
Expanded Access
Expanded access (also known as early access, compassionate use, right to try, or managed access) may be an option for people with serious or life-threatening conditions to access investigational therapies outside of a clinical trial when other treatment options have been exhausted.
At Genascence, we believe clinical trials are the optimal route for developing investigational therapies to treat patients. Through clinical trials we are able to demonstrate the safety and efficacy of GNSC-001 in a controlled environment. This is in order to produce data to meet rigorous regulatory standards set by the U.S. Food and Drug Administration, the entity responsible for ensuring the safety of clinical trial participants and for the assessment and approval of new drugs in the U.S. Therefore, Genascence does not currently offer an expanded access program for GNSC-001 anywhere in the world.
For information about U.S. clinical trials including information about disease area, study type, location, contact information, and recruitment status, visit ClinicalTrials.gov.